lewis structure of pf2cl3|What is the Lewis structure of [//substance:PF2Cl3//]? : Tuguegarao Now in the above sketch of PF2Cl3 molecule, put the two electrons (i.e electron pair) between each phosphorus-fluorine and phosphorus-chlorine atoms to represent a chemical bond between them. These . Tingnan ang higit pa 3:30 pm 15:30 in MDT is 5:30 pm 17:30 in EDT and is 5:30 am 05:30 in Manila, Philippines. MDT to EST call time . Best time for a conference call or a meeting is between 6am-8am in MDT which corresponds to 8pm-10pm in Manila. 3:30 pm 15:30 Mountain Daylight Time (MDT). Offset UTC -6:00 hours 5:30 pm 17:30 Eastern Daylight .
PH0 · What is the Lewis structure of [//substance:PF2Cl3//]?
PH1 · SOLVED: Draw the Lewis structure for PF2Cl3
PH2 · Phosphorus Difluoride Trichloride, PF2Cl3 Molecular Geometry
PH3 · PF2Cl3 Lewis structure
PH4 · PF2Cl3 Lewis Structure in 5 Steps (With Images)
PH5 · PF2Cl3 Lewis Structure & Characteristics: 13 Complete Facts
PH6 · Lewis Structure of PF2Cl3 (With 5 Simple Steps to Draw!)
PH7 · Lewis Structure of PF2Cl3 (With 5 Simple Steps to Draw!)
PH8 · Lewis Structure Finder
PH9 · 9.3: Drawing Lewis Structures
PH10 · 1.2: Lewis Structure
William Hill football betting; Ladbrokes football betting; Bet365 football betting; Unibet; Paddy Power football betting; Coral football betting; Betfred promotional code; Our Final Conclusion on Football Accumulator Tips. ACCAs are very popular among punters even if they re not experienced.
lewis structure of pf2cl3*******Lewis structure of PF2Cl3 contains a single bond between the Phosphorus-Fluorine atoms and Phosphorus-Chlorine atoms. The Phosphorus atom (P) is at the center and it is surrounded by 3 Chlorine atoms (Cl) and 2 Fluorine atoms (F). The Phosphorus atom does not have a lone pair while . Tingnan ang higit pa
Here, the given molecule is PF2Cl3. In order to draw the lewis structure of PF2Cl3, first of all you have to find the total . Tingnan ang higit paWhat is the Lewis structure of [//substance:PF2Cl3//]?While selecting the center atom, always put the least electronegative atom at the center. (Remember: Fluorine is the most electronegative element on the periodic tableand . Tingnan ang higit paNow in the above sketch of PF2Cl3 molecule, put the two electrons (i.e electron pair) between each phosphorus-fluorine and phosphorus-chlorine atoms to represent a chemical bond between them. These . Tingnan ang higit pa
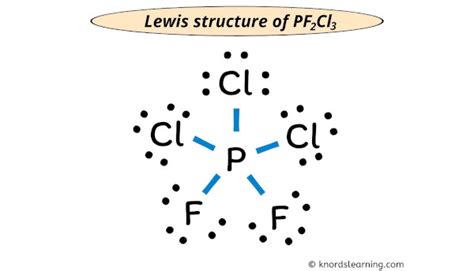
Lewis Structure is most simple representation of valence shell electrons in an atom. Let us study the Lewis structure of PF2Cl3. Calculate the total valence . PF2Cl3 lewis structure has a Phosphorus atom (P) at the center which is surrounded by two Fluorine atoms (F) and three Chlorine atoms (Cl). There are .
PF 2 Cl 3 has one phosphorus atom, two fluorine atoms, and three chlorine atoms. In PF 2 Cl 3 Lewis structure, there are five single bonds around the .Lewis Structure Finder. Added Jun 9, 2014 by Tester in Chemistry. This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha. Get .What is the Lewis structure of [//substance:PF2Cl3//]? Natural Language; Math Input; Extended Keyboard Examples Upload Random. Compute answers using Wolfram's .
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to .First draw the Lewis dot structure: Electron geometry: trigonal bipyramidal. Hybridization: sp 3 d. Then draw the 3D molecular structure using VSEPR rules: Click and drag the .
1. Determine the total number of valence electrons in the molecule. - Phosphorus (P) has 5 valence electrons. - Fluorine (F) has 7 valence electrons. - .lewis structure of pf2cl3 this is the complete Lewis structure of CO 2. For Lewis structure purposes, the lone-pairs can only be moved from terminal atoms to the central atom to form .
3. PF 3 Cl 2 lewis structure lone pairs. The non-bonded electrons are present in the valence orbital after the bond formation known as lone pairs. Let us count the lone pairs of the PF 3 Cl 2 molecule.. The total number of lone pairs present in the PF 3 Cl 2 molecule is 30 which means 15 pairs. Those are the contribution from the F and Cl site .
Chemistry questions and answers. 1 What is the total number of valence electrons in the Lewis structure of PF2Cl3 ? electrons (2) Draw a Lewis structure for PF2Cl3.1 What is the total number of valence electrons in the Lewis structure of NO2Cl ? electrons 2) Draw a Lewis structure for NO2Cl.What is the total number of valence electrons in the .lewis structure of pf2cl3 What is the Lewis structure of [//substance:PF2Cl3//]? The Lewis structure is a structure that shows the bonding between atoms as short lines (some books use pairs of dots), and non-bonding valence electrons as dots. 1.2.1 Lewis Structure of Diatomic Molecules. To learn about Lewis structures, we will start with the Lewis symbol. The Lewis symbol is the chemical symbol of an element with .
Lewis Structure Finder. Added Jun 9, 2014 by Tester in Chemistry. This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Draw a Lewis structure for PF3Cl2 and answer the following questions based on your drawing. 1. For the central phosphorus atom: . The number of lone pairs. =. The number of single bonds. =. The number of double bonds. PF2Cl3 Ángulo de estructura de Lewis. El ángulo formado entre los enlaces de un compuesto es su ángulo de enlace. Háganos saber sobre el ángulo de enlace de PF 2 Cl 3. El ángulo de enlace de PF2Cl3 es 900 y séptima0. El ángulo de enlace no es el mismo para todos los enlaces. Los bonos ecuatoriales tienen un ángulo de 1200 y .
A step-by-step explanation of how to draw the PF3Cl2 Lewis Dot Structure.For the PF3Cl2 structure use the periodic table to find the total number of valence . The Lewis structure of PCl3 consists of one phosphorus atom (P) and three chlorine atoms (Cl). Phosphorus has five valence electrons, while chlorine has seven. Therefore, the total number of valence electrons in PCl3 is: 5 (phosphorus) + 3 x 7 (chlorine) = 26 valence electrons. To distribute these electrons, we place three chlorine .
The concept of covalent bond was introduced by the scientist G.N Lewis in 1916.The molecular geometry of PF₂Cl₃ is trigonal bipyramidal.. What is Lewis structure? In Lewis structure, dots represent electrons.Such structures are referred to as Lewis dot structures or simply Lewis structures. Steps of drawing AlF3 lewis structure Step 1: Find the total valence electrons in AlF3 molecule. In order to find the total valence electrons in an AlF3 molecule, first of all you should know the valence electrons present in aluminum atom as well as fluorine atom. (Valence electrons are the electrons that are present in the outermost .What is the Lewis structure of [//substance:PF2Cl3//]? Natural Language; Math Input; Extended Keyboard Examples Upload Random. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, . Lewis structure of PF3Cl2 contains a single bond between the Phosphorus-Chlorine atoms and Phosphorus-Fluorine atoms. The Phosphorus atom (P) is at the center and it is surrounded by 3 Fluorine atoms (F) and 2 Chlorine atoms (Cl). The Phosphorus atom does not have a lone pair while the fluorine & chlorine atoms have . 6 Steps to Draw the Lewis Structure of PF2- ion Step #1: Calculate the total number of valence electrons. Here, the given ion is PF2- ion. In order to draw the lewis structure of PF2- ion, first of all you have to find the total number of valence electrons present in the PF2- ion. (Valence electrons are the number of electrons present in the .
SF 5 Cl Lewis structure. SF 5 Cl (sulfur chloride pentafluoride) has one sulfur atom, five fluorine atoms, and one chlorine atom. In SF 5 Cl Lewis structure, there are six single bonds around the sulfur atom, with one chlorine atom and five fluorine atoms attached to it. And on chlorine and each fluorine atom, there are three lone pairs.PCl5 Lewis Structure Step-by-Step Guide. 1. Determine the total number of valence electrons in PCl5. Phosphorus (P) is in Group 5 of the periodic table, so it has 5 valence electrons. Chlorine (Cl) is in Group 7, so it has 7 valence electrons. Since there are five chlorine atoms, the total number of valence electrons is 5 (from P) + 7 (from .
Step 1. Lewis Structures are used to describe the covalent bonding in molecules and ions. Draw a Lewis structure for PF2Cl3 and answer the following questions based on your drawing. 1. For the central phosphorus atom: The number of lone pairs = The number of single bonds = The number of double bonds = 2.

Lewis structure of pf2cl3. For the C2H2Br2 Lewis structure, calculate the total number of valence. Pf3br2 Lewis Structure_Yaelp Search. it provides the lewis structure,molecular geometry, and bond angle of PF3Cl2 and PF2Cl3. Lewis structures are useful when the centralatom is bonded to up to four other atoms.VIDEO ANSWER: We were going to have the Lewis structure for Part eight. We need to have the structure of your four. The balanced electron is eight electrons. Seven electrons are the violence electrons. We have X. E., so we will have their electrons.
View deals, see photos and read reviews for the best Israel hotels from travelers like you - then compare today's prices from up to 200 sites on Tripadvisor.
lewis structure of pf2cl3|What is the Lewis structure of [//substance:PF2Cl3//]?